Dr. Raul Poblete Silva F.A.C.S.
Emeritus Assistant Professor, Department of Surgery, Universidad de
Chile, former Head Department of Vascular Surgery, Hospital Militar de
Santiago,
Carlos A. Selmonosky MD, F.A.C.S., F.A.C.C.P.
Department of Medicine (Visiting Faculty) Inova Fairfax Hospital,
Falls Church, VA, USA.
E-mail address: Dr Raul Poblete S. raulpobletesilva@hotmail.com
DEFINITION
The Thoracic Outlet Syndrome (TOS) is a disease characterized by the
presence of heterogeneous symptoms that individually can be
predominantly neurologic and or arterial and or venous that often affect the upper extremities. The symptoms, mainly pain, are also frequently located in the head, neck, shoulders and the upper thorax (1,2).
Due to the fact that both vascular and neurological systems are affected with different degrees and combinations of symptoms, it has not been an easy task to define this multi symptomatic and polymorphic disease as a clear cut pathology.
Therefore serious follow up have not been performed and it has been a difficult task to understand the disease as a whole.
Actually it is not widely known and it is also a controversial issue for some physicians. Moreover, it is sometimes strongly denied by those who have not had the opportunity of identifying it as a disease or even when they have not dealt with TOS patients. Sometimes, due to all the facts mentioned above, we have had to deal with the complications of the untreated TOS.
We are confronted with a disease that is commonly undiagnosed by
the majority of physicians.
Diagnosis of TOS is mainly done on clinical basis (3), therefore when TOS is clinically suspected diagnostic workup should continue with chest and cervical spine disease X-rays, to rule out diseases that may imitate the symptoms of TOS.
The majority of TOS patients have bilateral symptoms, although one side usually is more affected (4-6).
ANATOMICAL BASIS OF TOS
In children and teenagers the only significant anatomical aspect that
reveals the presence of TOS is the radiological sign showing that the
first´s ribs are less curved and look more like a open arch pointing down and not the hypertrophy of the anterior scalenus muscle which is typical of adults TOS.
In addition, the scalene is usually normal because it will get hypertrophied only in adulthood. We suspect that the permanent traction exerted on the superior border of the first rib elicited a progressive bone tissue generation which could explain the widening of the first rib especially in the anterior arch, therefore young adults already has a sickle shape aspect. This can increase to a boomerang shape which is a very frequent finding in the adult TOS. The first rib in these cases is widened and it will compress from down upwards the neurovascular bundle of the upper extremity, adding the transversal forces exerted by the hypertrophied anterior scalenus muscle.
That is why the neurovascular compressions in the adult, and even more in the longstanding ones, have a mixed origin: first the hypertrophied or broadened first rib exerts an upward compression, they will commonly compress the lower elements of the brachial plexus, and the C8-T1 cords will suffer the most. Second, the hypertrophic anterior scalenus exerts a transversal compression backwards affecting both the subclavian arteries and the brachial plexus, and anteriorly the
subclavian vein, very close to the point where the jugular vein joins the subclavian vein (7-10).
It is also worthwhile to remember that the cephalic and cervical venous
system have no valves that would stop or dampen abnormal venous blood flow and although the internal jugular veins have valves at the jugular bulb at its junction with the subclavian vein, they are frequently anatomically or functionally incompetent.
It is also noteworthy that the hypertrophied and contracted anterior scalenus muscle exerts a strong although intermittent compression of the vertebral artery, causing in severe TOS diverse symptoms that are very characteristic of vertebrobasilary insufficiency. Because the vascular arterial tests are performed with the patient in recumbent position with the neck muscles relaxed, the intermittent vertebral compression is often overlooked.
To detect a potentially serious vertebral artery compression, we have used a simple and reliable method using a pocket doppler device and asking at the same time the patient to flex his head while forcing his chin upwards, therefore dynamic flow variations become evident.
The above mentioned altered anatomical structures such as widened ribs associated with hypertrophied scalenus muscles have been a constant finding in over 300 operated TOS patients and clearly account for most of the TOS symptoms and the altered vascular laboratory findings.
Curiously enough these facts have been for years mostly overlooked, because
attention was focused in other well-known facts, such as the existing cervical ribs or enlarged transverse processes from which musculofibrotic bands arise compressing the neurovascular bundle. These bands were described in detail by Roos (11), although it should be pointed that an recent anatomical research to examine the interscalenic triangle anomalies have shown their incidence may be less than usually though (12). The old rib fractures with large calluses as a compressive element have also been discarded.
CLINICAL ASPECTS OF TOS
Neurological Symptoms:
Neurological symptoms are present in almost all TOS patient in varying degrees,
sometimes pain is one the outstanding symptoms, that can resemble herniated cervical discs. They are the result of a compression of one or more branches of the brachial plexus (13). Most are affected with recurrent pain that may become permanent or get worse when the arms are elevated.
Muscle complaints may even lead to a diagnosis of myositis, dorsallis fasciitis or
even temporomaxillary pains and dysfunction, recurrent arm or shoulder tendinitis or scapular related pains.
They can mimic pains of diverse entrapment neuropathies of the upper limbs at the elbow and or wrists, and also they can mimic radicular pain, the interscapular area can also be the site of pain.
Chest pain may mimic anginal pain and women may feel breast discomfort or pain. This symptoms apparently unrelated to TOS are believed to arise from the compression of the sympathetic fibers integrated into the nerve roots C8-T1 of the brachial plexus and whose connections are shared with the heart sympathetic nerves (14-18). When transmitted to the brain and coming from the same dermatome will cause this peculiar thoracic pain.
Pain of the upper limbs and neck is usually constant or may be replaced by paresthesias and heavy arms sensations. At times symptoms are worse at night. During daytime pain appears when raising the arm as when reading the newspaper, combing with the use of hair dryers, or any other activity that needs arms lifted for a while.
The most inferiorly located cord of the brachial plexus is the medial cord that give origin to the ulnar nerve, the fourth and fifth fingers paresthesias or numbness reflect the compression of the roots C8-T1.
The median nerve will give paresthesias to the middle fingers, rarely the thumb is affected, and when this occurs it means that the uppermost fibers of the brachial plexus were involved due to compression by scalene muscle hypertrophy. Then a differential diagnosis with Carpal Tunnel Syndrome is indicated.
Motor disturbances. Progressive motor disturbances affect the whole hand, and specially the 5th digit loosing the abduction and adduction capacities completely, until fine motor control is severely impaired and even a fine tremor may appear.
TOS patients frequently complain of intense cold fingers (19), nose and ears, this during day hours (Raynaud´s Phenomenon). Visual symptoms as increasing focusing difficulties and also diplopic and blurred vision are not infrequent occurences. Frequent headaches, some with migraineous character may be present.
Probably all this to compression at the thoracic outlet level of the sympathetic fibers that comprise up to 15% of the branches of the brachial plexus in predominantly complicated neural TOS.
Left untreated a severe progressive denervation of the upper limb occurs affecting mostly the C8-T1 nerve areas. The muscle atrophy can lead to advanced muscle wasting of hand and arms results in ankylosis of the shoulder that may lead to a frozen shoulder.
Arterial Symptoms:
It is usual to observe skin color changes when arms are lifted up or lowered. These changes are caused by postural compression of the subclavian arteries, though it needs a strong compressive effect to significantly reduce the blood flow.
Postural vertigo and balance difficulties occurs frequently in many TOS patients even in teenage patients and they have a progressive course causing falls and drop attacks. Recurrent diplopic episodes and frequent syncope may be present. Many of these episodes are classified as vasovagal, but they do not react to midodrine treatment, and they usually disappeared after TOS surgical treatment.
All of the above mentioned array of symptoms are caused by the intermittent
compressions by the hypertrophied scalene muscles and fibrous bands which exert pressure on the vertebral artery (20-23).
Intermittent tinnitus due to non linear blood flow to the inner ear appear creating false othological signals. In occasions inner ear microembolism with emboli originated in the compressed vertebral arteries causes cochlear infarcts, producing sudden hearing loss which may become permanent. If a cochlear embolism is suspected it should be treated promptly with antiplatelets drugs, and in early treated cases they may be totally reverted.
Some other othological considerations. Kimura (24) using monkeys provoqued
selective cochlear arterial embolisms and he could localized which level
of the cochlea was affected by the different embolizations. For unknown reasons cochlear microembolisms tend to occlude the cochlear branch producing sudden
hearing loss but only affecting the high frequencies.
Other embolic processes affect the upper limbs circulation and are usually recurrent, most of them microembolism of the fingers which turn cyanotic and painful. Fortunately they are often self limiting and readily react to antiplatelets treatment (25-27).
A seldom encountered embolism is that one that affects both the right
arm and ipsilatered brain hemisphere. The origin is a subclavian artery
thrombus located exactly at the level of maximal compression. The thrombus
extend to the brachiocephalic artery then migrates both to the common right carotid artery as well to the subclavian artery. This causes ischemic changes of both the right upper extremity and symptoms of right cerebral hemispheric deficit appear.
Venous Symptoms:
Venous symptoms though frequent are often overlooked for the inexperienced observer for they are not so evident as the neural or arterial compromise.
The following symptoms are frequently seen: venous enlargement of arms
and hands with some edematous swelling most usually seen when awakening. Patients complain that their fingers do not feel right, they can not take their rings off, minor "spontaneous" venous rupture in the hands and fingers may sometimes occur. They may be recurrent and only reflect the existing venous hypertension caused by some degree of stenosis thrombotic or positional of the axilary-subclavian veins.
Venous TOS complications are superficial and deep thrombosis that may appear in young women free from thrombophilic diseases but using strogenic or anticonceptive pills. In opportunities a rare massive deep venous thrombosis of the upper extremity which is also known as effort venous thrombosis or Paget-Schroetter´s Syndrome (28). This can be a severe complication of an underlying TOS.
PHYSICAL EXAMINATION
To adequately diagnose TOS the examiner should have a basic knowledge of the complex and polymorphic symptomatology that will evoke the possibility of a TOS.
Diagnostic aids should be used as a chest and cervical spine x-rays, with a clear view of the first rib and the transverse process of the seventh cervical vertebra. A complete vascular laboratory is very important to objectively demonstrate the existence of subclavian vascular arterial compression and its magnitude, and also the presence of sympathetic hyperactivity resulting in digital and hand vasoconstriction.
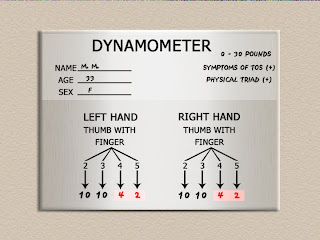
A very useful aide to the physical examination in our experience is the Selmonosky´s diagnostic triad (29) which according to the author is present only in TOS and not in any other compressive neuropathies of the upper extremities.
The Selmonosky´s triad consists of :
1.- Observe tiredness, or and numbness and paresthesias in the arms and hands, or and color changes of the hands, or venous dilation maintained when arms are raised up over the head for 3 minutes at time one. One hand or both may turn extremely white and this is known as the White Hand Sign.
In venous type TOS-MS patients the veins do not dilate except for intense
jugular dilatation during inspiration when the patient is in the supine position.
2.- Note the weakness during abduction and adduction of the fifth finger, also testing the fifth finger pulp digital pinch strength when opposing the thumb. Comparing the fifth finger strength with the second finger pulp digital strength when opposing the thumb.
3.- Note if pain or tenderness is elicited when pressure is applied with the examiners thumb to the supraclavicular areas just lateral to the sternocleidomastoid muscle. Nonverbal response, particularly the facial expression should be noted to assess the pain.
Scalene muscles hypertrophy is present in almost all the adult patients
and they can grow to be very thick, scalene muscle contracture is of
usual occurrence and can be easily detected. Many patients have a
slouched posture that may reflect depression and cases with severe
pain may present a typical posture with splinting of the upper extremities.
Raynaud´s Phenomenon, especially in venous TOS, is a frequent finding with a medium to great intensity. Hands and fingers will appear dry and cold, some may exhibit intense sweating.
DIAGNOSING TOS
Classical clinical examination:
- The pulse in elevated arm positions are many times decreased in so called
normal subjects, and they should not be used as a sole diagnostic test diagnostic of TOS unless other signs of neurological and venous compression are present.
The predominant Neural and Venous type TOS comprise the majority of TOS
syndromes. These positional tests will only appear useful when the compression on the subclavian arteries is extreme, and become of value only in the arterial type TOS when an easily perceptible decreased in the pulse is clearly felt as the arm is elevated and it may even be feel as a pulseless arm.
- Electrodiagnostic neurological tests:
Along these years we began using the classical EMG followed by diverse
nerve conduction studies, even evoked potentials were tried
following the neurologists advice, we used them routinely as preoperative base line to confirm TOS (30). But EMG failed as a confirming test and they did not work either as a pre or postoperative diagnostic or prognostic tests.
In other cases EMG only suggested peripheral nerve entrapment, such a
ulnar nerve entrapment or a carpal tunnel syndrome in patients that never suffered from these conditions. Thinking and performing a retrospective analysis, is clear that a TOS compression may in some patients cause nerve damage but only after some years of intense compression.
We also learned what happen with those patients who preoperatively had some form of abnormal electrodiagnostic neurological tests. Not less than 5 years after the TOS operation we have noticed no changes in the tests, although the patients clinically felt well and many were totally asymptomatic; it is well known fact that the regeneration of nerve fibers is a very slow process.
For the already mentioned reasons we have abandoned the routine use of neurological electrodiagnostic studies and, in accordance with our neurosurgeons, we use them only in cases of simultaneous occurrence of a low cervical disc herniation and a TOS or a suspicion of other peripheral upper extremity neuropathies.
As a conclusion there are not currently electrophysiological methods useful in the TOS diagnosis.
- Radiology:
A chest AP-X ray picture and AP cervical views are absolutely necessary and useful to view and asses the shape and position of first ribs, the shape and position of clavicles, and the shape and length of the transverse processes of C7, and finally rule to out any other pathology that may imitate TOS symptoms.
Other modern diagnostic techniques such as computerized tomography and its latest upgrade helicoidally computerized tomography, angio MRI and neurography techniques may all be helpful but, until now, in rather exceptional occasions. They may be invasive due to the use of contrast media, and the total cost of diagnosing increases substantially.
When required the usual transversal planes (axial tomography) only limited for TOS purposes it is necessary then to use oblique imaging so as to adequately show the thoracic outlet structures
- Brain Pet Scan:
Dr. Fernández Noda in Puerto Rico has worked with Pet Scans to evaluate
cerebral circulation in some Parkinson disease cases who he thought could be caused by vertebral artery intermittent compression due to scalenus muscles hypertrophy.
We have used this method only in a few cases in which we needed a more
detail information of the brain circulation. We used this method in some TOS associated with some syndromes such as Asperger´s disease, among others, and we found only an irregular distribution of the isotope which left some areas as the basal ganglions with a minimal flow, and digital substraction angiography showed the normal permeability of those vessels.
We could also observe a complete normalization of the circulatory anomaly after TOS was operated upon in the same way that the brain condition got better. This
suggested that this neurological abnormality could represent only a circulatory imbalance.
- Arteriography and phlebography:
Conventional and positional studies are seldom used at present due to their
potential invasive risks; years ago when we begin to systematically studied TOS we used them routinely in almost all TOS cases.
The first angiographies were done though femoral catheterization reaching the origin of the subclavian artery where contrast media was injected depicting the angiographic features of the subclavian arteries and its branches. By moving and abducting the arm while the procedure was performed, we could observe the precise positional location of the compressions and also the intermittent character, which otherwise would have not been demonstrated. We could also even determine if compression was due to the rib, the scalenus muscle, or both.
All this radiology images were extremely useful and necessary in those years, but we missed some other methods that could show the hemodynamic effects.
We used these methods for years for their valuable information both to confirm the TOS presence but also to assess its severity. We did also learn that the great majority of TOS were of the predominant neurologic type, and as anatomy teachers of the brachial plexus we showed that their components run
in parallel course under the subclavian artery, as a two barrel shot
gun. Due to this fact we concluded that all relevant arterial compression (shown by arteriography) would necessary affect the neural component in a very similar degree.
- Conventional phlebography:
They were used to examine the predominantly venous TOS. The contrast media
was injected in the arm or hand veins distal to a ligature and the ascent of contrast through the deep veins could be clearly shown and how it stopped at the anterior scalenus muscle level in a characteristic place which correspond to the tubercle of the first rib where the anterior scalene muscle inserts.
We could also see that in all cases of predominant venous TOS there were different degrees of venous reflux towards the superficial veins of the hand, but not to the jugular veins.
As we wanted to localize the compression, specially the intermittent ones, we could establish the presence of venous thrombosis or positional hypertension in the subclavian veins in cases of superficial and deep venous thrombosis of the upper limbs specially in the recurrent cases and in absence of thrombophylias.
Vascular laboratory studies:
Segmental pressure studies:
They are very useful to perform segmental distal and proximal systolic pressures in the arms and forearms at rest to check if distal pressures are higher than proximal as in normal patients. When the ratio is inverted in the neutral position, arterial compression must be suspected. This is one element of positional arterial
compression that must be taken in account and consider with the other vascular tests.
- Pulse wave recording:
It is also of great interest to have a detailed recording of pulse waves along the subclavian, brachial, radial, cubital, palmar arch and all digital arteries. The typical finding in TOS is a sustained unilateral or bilateral vasoconstriction of the digital arteries objectively demonstrated by digital plethysmography. They should not be used as a sole test to make a diagnosis of TOS.
- Plethysmography and positional photoplethysmography:
Both tests combined are the best non invasive way to establish the existence of an intermittent arterial compression of the subclavian artery due to TOS of any type. Besides it is a simple and objective way to evaluate its severity. All our patients were routinely studied during many years in vascular laboratories with plethysmography.
The plethysmographic analysis of arms and hands in the rest position has always been of great value in cases of atherosclerotic changes to rule out fixed atherosclerotic disease, subclavian or axillary involvement, but in TOS the arterial compression is positional and they were generally non diagnostic.
The positional plethysmography measures digital flow differences between rest and during various positions of the upper extremities or neck, in both index fingers, with piezoelectric elastic bands (impedance plethysmography).
If it is well done allows to make evident minor or moderate arterial compressions.
We used it for many years in Chile, in other countries it is banned because is not
reimbursed for the insurance companies. For this reason some others use pneumoplethysmography which measures volume variations within air cuffs and both obtain credible results.
It is the best non invasive way of objectively demonstrate the degree of compression in those TOS with arterial predominance.
TOS studies using positional digital photopletysmography to show subclavian artery compression are essential in almost all TOS patients. In the resting position we will find a digital wave morphology characteristic or similar to Raynaud´s Phenomenon, being this finding highly suggestive of TOS.
Raynaud waves have different amplitude at different digits and an evident asymmetry in the same waves with great differences in its ascending and descending branches.
Digital vasoconstriction is frequently present in TOS, regardless its severity and clinical, form because is produced by the compression of the sympathetic fibers which add up to 15% of the brachial plexus fibers.
When Raynaud´s waves appear in absence of a proven connective tissue
pathology the probabilities of TOS are very high.
The study is performed simultaneously in both sides and beginning with the resting position to obtain a baseline. Afterwards, the study continues with diverse positions of the upper extremities as when the patient is asked to raise the arms 90º or 180º and the so called costoclavicular posture.
In extreme arterial compression the flow may totally be suppressed in one or another positions even at rest, but in general the arterial compressive effect appear evident when arms are lifted 90º to 180.
In cases of mainly subclavian compression by a anomalous or broadened
first rib, scalenus muscle or bands, we will find usually a slight reduction in the
neutral posture digital flows, but frequently absence of flow in the 90-180° abduction position in one or both sides. It is remarkable also the reduction during the costoclavicular tests.
- Color Echo Doppler:
We did not routinely used them in classical TOS because we thought that although is very useful in those with predominant arterial and venous manifestations, it shows less information in the more frequent neural TOS patients. When well accomplished by vascular technicians well informed of the TOS anatomy and pathology, it is possible to establish with accuracy, the flow speed in both subclavian arteries and veins at rest or with positional maneuvers, to objectively document the positional compressions.
These tests are irreplaceable as a non invasive study of TOS-MS related patients, because they are mainly venous. Moreover is necessary to clearly define if there is some jugular vein reflux, because of the new findings in the theories of vascular neural origin of MS.
- Transcranial Echo Doppler:
Some years ago in classical in studies of TOS patients its results were not objective, accurate or reproducible as we thought so, we discontinue this study. At present we have no experience in MS patients with this technique used by Dr. P. Zamboni to study venous cerebrospinal disturbances.
TOS incidence and its apparent relation to multiple sclerosis
TOS real incidence in the general population is not known due to the absence of widely recognized signs or cost effective laboratory tests, and the lack of sufficient diffusion of the syndrome in the medical literature, it is also a poorly defined medical entity. The actual incidence seems low in some studies, and varies from 1 (31) to 80.000 (32) per million of population. In more recent studies the incidence appears to be higher, it is an often misdiagnosed cause of chest (33-35), neck and shoulder pain (36) and one of the frequent upper extremity neuropathies related to sport (37).
Moreover, we wish to give an account of some recent findings that point to a close relation between TOS and MS and could signify important changes of our present knowledge of both (TOS and MS) entities.
In recent months 85 MS patients suffering from the diverse types and with different degrees of CNS involvement, were studied clinically and submitted to a complete vascular laboratory workup. All these patients had an undiagnosed TOS, unilateral or bilateral, and usually a TOS of predominantly venous type.
The second surprising finding was that all the MS patients without exception presented a complicated TOS, because they all had unilateral or bilateral jugular retrograde blood flow inversion. This abnormal jugular blood flow could be moderate to very intense and, up to our present knowledge, we understand that this is an overtly abnormal situation and definitely pathological.
This is remarkable, because we have had so far never found jugular vein retrograde reflux in cases of TOS patients not suffering from MS.
Furthermore, suspecting that MS patients could also have TOS, all patients underwent extensive vascular studies which confirmed the abnormal retrograde jugular vein flow.
In related literature reports (38, 39) some authors have suggested that a cerebral abnormal venous flow mainly jugular, and on occasions also via acygos veins, creates cerebral and cervical cord venous hypertension. This is a condition that alter capillarity permeability together with the liberation of free radicals that modifies iron metabolism. This condition has been associated with MS and deemed responsible for demyelization and plaque formation both at the brain and cervical cord levels (40-42).
This is an attractive and totally new theory that is contrary to the usual immunological theory of MS. An additional anatomical fact of the craniocervical venous drainage is the true “absence” of venous valves in the high portion of the jugular veins. Although the inferior bulb of the internal jugular has valves, frequently they are anatomically or physiologically incompetent. These allow the free transmission of the more proximal venous pressure variations backwards to the craniocervical segments.
Thus an intermittent or permanent hypertension by compression or occlusion of the distal jugular or subclavian vein is directly transmitted to the capillary beds of brain and spinal cord.
All the above mentioned facts, and the recent and most interesting publications by Dr. Paolo Zamboni, who by using diverse methods, could confirm that cerebrovascular abnormal retrograde jugular flow was a constant finding in MS patients.
This very important finding, which was until then considered only a theoretical possibility, was now a demonstrable reality. Based on his findings he clearly stated that the venous reflux was caused by the constant presence of unilateral or bilateral jugular venous stenosis.
After studying 65 MS patients he showed that all of them had jugular reflux, due to some form of venous stenosis which he named as "jugular vein stenosis of unknown cause" and which were responsible of a condition he named as "chronic cerebrospinal venous insufficiency" denomination which we think is not
entirely appropriate because what the stenosis really provoque is a true venous cerebrovascular hypertension.
The original denomination has prevailed and is known as CCSVI.
In Dr. Zamboni’s outstanding original report he describes having studied his MS patients with echo doppler, venous MRI or conventional phlebography and serial venous pressure recordings along the jugular veins (43), whereby he concluded that the majority of the venous stenosis affected the jugular veins and only a few the azygos vein. He also suggested that they should be treated by angioplasty via the inguinofemoral route, as the only vascular procedure he recommended in
his initial series.
So he exposed a new vascular theory opposite to the exclusive immunologic origin of MS. He reported this treatment favorably modified the clinical course of the intermittent recurrent types of MS, although this was not the case in the primary and secondary progressive forms. Beside he could also observe that about half of the patients developed venous restenosis in the angioplasty area and with the intent to avoid the restenosis, he suggested placing stents as an logic alternative to prevent it.
This experience was replicated in some others endovascular centers and this could verify that in 90 to 84% % of the studies (and only 7 and 24% in controls) (44, 45) the existence of jugular venous reflux and a venous cerebral vascular hypertension was confirmed.
It is worthwhile noticing that in many of these centers some unwanted events related with stents, such as displacement has caused serious complications.
Our surprising findings
Some years ago relying in our experience in venous angiography in TOS (46) we could observe that the injected contrast media stopped exactly at the junction of the anterior scalenus muscle and the first rib.
Our primary interest was to confirm the diagnosis of thoracic outlet venous compression but and additionally we could seen an important venous reflux towards the superficial venous circulation reaching the hand. We even used isotopic venography which showed basically the same although not so clear as the radiological contrast studies described above.
Dr. Zamboni’s idea was that the jugular vein circulation was clearly blocked at the lower level of the vein, but he did not identify that this block occurs precisely at the level of the thoracic outlet.
One of the authors (R.P.S.) managed to put findings together with his previous experience in TOS diagnostic aides (contrast venography and others). Adding his decades of TOS surgical experience, he concluded that all those venous stenosis, until now described of unknown origin, were caused in many MS patients by an undiagnosed severe TOS of the predominant venous type. It also affected all the neurovascular bundle in different degrees.
These finding appeared in all the cases of these MS patients whom suffered from a severe TOS usually of venous predominance, they were examined and studied
as thoroughly as possible, and they showed a considerable jugular retrograde venous reflux.
These findings led to the idea that all patients presenting both TOS and jugular vein reflux should be considered complicated cases, and for this reason not amenable to conservative treatment which has been so helpful in the treatment of TOS patients without MS.
Based on all these facts we realized that the idea of surgical decompression of the neurovascular bundle would clear the TOS typical symptoms and also could slowly revert the internal jugular vein inverted flow, thereby eliminating the compression of the jugular and subclavian veins and relieving the venous hypertension upon the cephalic and cervical venous territories including the venous brain bed.
The surgical approach was Dr Roos’s approach, that is via an axillary route
dissecting upwards making an anterior scalenotomy and identifying and
resecting the anterior arch of the first rib. Dr. Roos’s approach was preferred because, in our large experience, is the one with excellent results and it is the same operation performed in non MS patients. We thought that Roos’s approach resolved with only one operation both the TOS symptoms and the venous flow anomalies.
TOS Clinical Aspects and peculiarities in presence of MS
We have reexamined all the symptoms and their variations in all the different clinical forms of TOS that we have studied during many years, and we have compared with those symptoms observed in different MS patients whose diagnosis was supported clinically and by MRI. These patients have been recently operated upon.
But what is interesting and is a surprising fact is that, quite a number of symptoms, were identical or could be MS symptoms that overlapped with those of TOS. According to our experience TOS and MS have each their own features, but these features can make diagnosis of the TOS a difficult task for the not well informed examiner.
Although without the same experience in MS, we have concluded that both TOS and MS share quite an important number of symptoms specially those involving the upper extremities, neck, head and chest. We have found that MS and TOS symptoms may coexist, and there is a tendency to attribute these symptoms to MS ,but as far as we know these have been characteristic features of TOS patients free of MS.
There are several quite constant conditions that might lead to diagnostic pitfalls. It is clinically help to remember that MS give patients and increased and generalized pain threshold that sometimes make very difficult the TOS diagnosis.
MS is suspected when symptoms of muscle weakens and early fatigability are
present and also lower extremities symptoms and bladder trouble which are nor seen in TOS.
Tremors similar to the extrapiramidal syndromes or chorea, hypertonicity and ataxia, are seen at times in non MS-TOS patients. Mild tremor of the upper extremities have disappeared fast post operatively. Unbalance, postural, visual symptoms and typical TOS posture are problems presents in both MS and TOS, but are by far more severe in MS, where they may lead to neural compromise and all the complications. This serious neural consequences do not occur in TOS without MS.
Fifth digit weakness is an essential finding in all neurologic type of TOS and is a common finding in all MS patients that we have treated, but it is not a typical MS feature and represent the presence of an under lying TOS compressive effect on the brachial plexus.
TOS characteristically gives C8-T1 roots symptoms which help to distinguish it from other compression neuropathies of the upper limbs, and also from other degenerative more diffuse diseases.
Evident and severe Raynaud´s syndrome is a constant finding in MS plus TOS patients, which have been confirmed in vascular laboratories with digital photopletysmography.
In TOS with MS the veins show a well defined feature. There is an important jugular retrograde reflux and the superficial veins of upper extremities are see down dilated but are usually thin.
TOS surgery:
Anterior scalenotomy.
During last year's we have seen wide surgical alternatives trying to correct this condition even before its causes, clinical forms and complications were well established.
Initially it was suggested to liberate the neurovascular bundle at the bottom of the neck with anterior scalenotomy with different ranges of success. Some still use this approach but not exclusively, as it was promptly noted that it is possible, postoperatively, that reinsertion to the first rib may occur, so it was advised to resect a segment to avoid this and the reappearance of the symptoms.
Afterwards, specially when the suspicion that cervical ribs bands or anomalous fibrous bands inserted in the scalene tubercle of the first rib play a role in TOS, diverse transverse cervical approaches were considered.
Supraclavicular route
The supraclavicular route was suggested in first place to section the scalene
insertion in its tubercle. After Roos publications, that anomalous fibrous bands can produces TOS, and more recently the resection of very wide or anomalous ribs it has been widely used.
The anterior scalenotomy through a supraclavicular access is feasible but is very difficult and risky to realize a costal resection in a someway blind approach to the cervical or costoclavicular structures. Brachial plexus trauma may be more frequent with this approach.
Never less, the supraclavicular route allowed a good access in those cases where in addition to correct a TOS it is necessary to resects some distal aneurismatic segment of the subclavian artery with or without prosthetic replacement as necessary in this long term cases or complicated ones (47-50).
Subclavicular access.
Afterwards it was used as transversal accesses downwards following the 2nd intercostal space. Through is was required an open thorax it offers an excellent view of the different structures of the costoclavicular space in order to decompress, resects or replace some arterial or venous segments. It’s the adequate route to control surgical vascular emergencies which may arise in TOS surgery or complex vascular trauma in the costoclavicular area.
Posterior approach.
Via an aciform incision surrounding the scapula which is lifted giving excellent view of first rib posterior arch (51). Besides this, it leaves a very visible scar and a scapular sequel almost impossible to recover. We have used this approach only to extirpate highly located rib tumors, its use has been indicated in reoperations in extremely difficult TOS cases.
Axillary route.
Finally Roos introduced the axillary route to operate TOS (52) in order to access directly to the anterior scalenus and the the first rib, and intending to obtain visual control of the neurovascular structures of the TOS area.
We regard it as the particularly adequate to obtain long lasting results for several reasons (53). Direct vision of the neurovascular bundle minimizing risks, the scalene muscle is separated from its rib insertion under direct vision and following by extraperiosteal resection of the anterior costal arch with the same advantages.
Resection of the anterior arc of first rib suppress prevent anterior scalenus reinsertion and eliminate definitely subclavian artery and vein compression and eradicate the inferior neural compression of the brachial plexus.
Our experience with this purpose suggest that no other surgical maneuvers are further needed, and the following should not be pursued for dangerous: too much broadening of the costal resection, medium or posterior scalenus resection, length vascular liberation, greatest brachial plexus nerve exposure or neurolysis of C8-T1 roots. We have not observed recurrences after decades of practice this technique if the periosteum of the anterior arch of first rib is removed, and we have seen that the chronic inflammatory involvement of the neurovascular components is slowly decreasing after surgery.
Sympathectomy.
Routine cervicothoracic sympathectomy is not used and only on rare occasions such as distal gangrene. Sympathectomy requires thoracic opening through the second or third intercostal spaces, for this we use the old lumbar sympathetic hooks instruments. Today, the thoracoscopic approach could be the method of choice.
The second and or third ganglia are removed, extreme care should be taken not to injure the upper half of the stellate ganglia so to avoid Horner’s Syndrome. The results of this additional procedures to TOS surgery are difficult to evaluate, nevertheless no amputations were needed in the cases it was used.
The role of venous angioplasties.
As predominant neural and arterial TOS comprise the majority of TOS types, venous angioplasties have no use in them.
Recently some venous predominant TOS complicated with deep venous thrombosis of the upper limb have been subjected, by some vascular surgeons, to venous angioplasties This was used to treat some forms of later stenosis of the subclavian veins, and have been successful, but stents or venous patches were also necessary.
At present and afterwards Zamboni experience it appears that venous angioplasties could be useful in dealing with stenosis and venous reflow in jugular and even acygos veins. We do not object the method per se, the reason is that angioplasty does not treat TOS, and apparently only in a short term, the chronic venous hypertension.
As we presume, the chronic fibrosis of the thoracic outlet is caused by longstanding, severe compression, mainly venous. This means that the existing
Thoracic Outlet compression has been undiagnosed and therefore untreated for a very long time causing the chronic pathological changes. These are mainly diffuse fibrotic changes in and around the thoracic outlet region, and contributing to the venous exogenous compression. Therefore, the best venous angioplasty with or without stent would be the one that could reduce jugular vein retrograde flow, and reducing the cerebral venous hypertension whereby modify the course of MS.
We cannot recommend primary venous angioplasty for patients with both
TOS and MS. We do recommend it for those patients who have reached a
general health compromise including respiratory deterioration and have
become a surgical risk.
Results of TOS Surgery.
As a general rule all TOS patients should receive physical therapy and postural exercises (54), it may include muscle relaxants such as ciclobenzaprine and
in the case of neural TOS with intense symptoms should may also have
pregabalin, at night.
With this treatment about 80 % of symptomatic patients with mild to moderate disease may expect good results, and some severe cases may also be relieved.
TOS patients who are finally operated are those who from the beginning had intense symptoms and also those whose conservative treatment was not successful. Our series of 250 operated TOS cases have had surprisingly good results. They experience sensation of well being and some of their symptoms have already vanished after 24 hours post operatively and thereafter continue to experience a progressive recovery.
Shortly after the operation the patients report absence of cervical pain, the headache and visual minor disturbances and hand paresthesias also disappear; head rotation is easier and has a wider range; the Raynaud Phenomenon disappears; motility and strengths of arm and hand begins to increase.
The residual symptoms in non MS-TOS patients diminish further with time and they are also related to adequate physical therapy. After two months the great majority of all operated TOS patients are entirely asymptomatic.
In the group of TOS patients complicated with MS that we have operated until now (we have 45 patients) with a short follow up, the immediate post operative recovery has not differed from all of those cases operated for TOS without MS, these patients recovery pattern has been constant throughout years of TOS surgical practice.
This fast recovery looks more related with the pure TOS symptoms rather than the MS itself. The striking difference is that in the TOS-MS complicated patients we have seen an early feeling of wellbeing in contrast with a general fatigue sensation that they had experienced for years.
A slow but progressive sensitivity recovery is evident on the proximal part of the inferior limbs, also with the abduction of the fingers and the dorsiflexion of the toes; the balance difficulties diminish and the gait improves.
These patients with TOS and MS have been operated randomly, that is to say no selection has been done considering the different types of TOS. Exceptionally some patients with severe respiratory advanced disease, or severe longstanding motility deterioration were not considered for surgical treatment, although some few ones with advanced neuromuscular deterioration were exceptionally operated.
These patients presented a remarkable initial improvement at least in their communicating and cognitive capacities and an initial strength recovery of the
upper limbs and specially the hands were felt much better, the toes dorsiflexion improved. Instability and gait have shown some improvement, although some patients are wheelchair bound, especially those with longstanding disease. Patients with neurogenic bladders have also reported some improvement,
mild tremors have disappeared.
The more severe involuntary movements remained unchanged. Many of the operated patients have continued their recovery with the help of constant and expert care of physiotherapists.
Summary and conclusions.
This is an update of our current knowledge and experience in the surgical treatment of TOS. The clinical aspects, pathology and complications of this disease are widely ignored and at times denied. Clinically it affects the upper limbs presenting with pain, ischemic phenomena, and venous congestion, all this may appear isolated or combined which may lead to diagnostic confusion, this is due to neurovascular bundle compression at the thoracic outlet triangles and the first rib upper edge.
Usually this situation is bilateral, but always more evident on one side, TOS may also present with more atypical symptoms as headaches, minor visual disturbances tinnitus of unexplained origin, or chest, neck and shoulder pain.
A detailed description of the vascular laboratory facilities is given, noteworthy are the different plethysmographyc evaluations and Echo Doppler that make evident jugular vein retrograde reflux.
Of the different surgical approaches to treat TOS, the transaxillary route of Roos has been our choice for many years, for it is safer. A transverse skin incision on the skin of the thoracic of the lower axilla opens the route to further upwards dissection that shall finally lead to the anterior arch of the first rib which, after careful dissection is partially extirpated liberating the pressure on the neurovascular structures.
Dr. Paolo Zamboni´s research and clinical experiences led us to determine the existence of jugular vein reflux in almost all TOS patients complicated with MS patients, and logically presuming proximal venous stenosis.
He treated these patients only with venous angioplasties, obtaining favorable results in some patients confirming his theory of venous hypertension as an important factor in the genesis of MS. The importance of his research and clinical experience have set a milestone in the research and treatment of TOS and MS and for the continuing scientific and clinical research in this apparently confusing subject of chronic cerebral venous hypertension.
TOS and MS.
Finally and to end this already extended update, it is necessary mentioning the TOS and MS connection established by Dr Raul Poblete Silva who managed to
put together his years of experience in dealing with TOS patients which included venous angiographies and several hundred operated cases, arriving to the conclusion that the jugular vein compression occurred at the thoracic outlet triangles configured by the hypertrophied scalene muscles, the first broadened rib and the fibromuscular bands, placing Thoracic Outlet Syndrome as a highly prominent and important factor in the genesis, and progression of Multiple Sclerosis.
REFERENCES
1.- Selmonosky C, Poblete R.
El diagnóstico del síndrome del opérculo torácico. Mitos y realidades.
Rev Chil Cir 2008; 60(3): 255-261.
2.- Poblete R.
Síndrome del opérculo torácico.
En Poblete R. Patología Arterial y Venosa, Sociedad de Cirujanos de Chile, A Yuri Ed., 1994: 479-503.
3.- Poblete R, Draper S, Velásquez A, Acuña R. Síndrome del Opérculo Torácico: Controversias sobre su diagnóstico y tratamiento.
Rev Chil Cardiol 1990; 9 (2): 75-85.
4.- Crawford F.
Thoracic outlet syndrome.
Surg Clin of NA 1980; 60(4): 947-956
5.- Machleder H.
Vascular Disease of the Upper Extremity and the Thoracic Outlet Syndrome.
In: Vascular Surgery, 2nd Ed, Grune & Stratton Inc., New York, U.S.A.,
1986: 683-702.
6.- Bahr J.
Síndrome del opérculo torácico. Aspectos vasculares.
Rev Chil Cirugía 1983; 35(1): 105-108.
7.- Edwards P, Moody P, Harris P.
First rib anomalies in association with cervical ribs: a cause for postoperative failure in the thoracic outlet syndrome.
Eur J Vasc Surg 1992; 6: 677-681.
8.- Hashimoto H, Nikaido Y, Kurokawa S, Miyamoto K, Sakaki T.
Thoracic outlet syndrome due to first rib anomaly: a case report.
No Shinkei Geka 1994 Nov; 22(11): 1063-1066.
9.- Yamaguchi R, Kohga H, Kurosaki M, Tamura M, Tosaka M,
Yashimoto Y.
Acute basilar artery occlusion in a patient with left subclavian artery occlusion due to first rib anomaly: case report.
Neurol Med Chir (Tokyo) 2008 Aug; 48(8): 355-358.
10.- Adesanya O.
Thoracic outlet syndrome secondary to first rib anomaly; the value of multi-slice CT in diagnosis and surgical planing.
Ir Med J 2007 Feb; 100(2): 377-379.
11.- Roos D.
Congenital Anomalies Associated with Thoracic Outlet Syndrome. Anatomy, Symptoms, Diagnosis and Treatment.
Am J Surg 1976; 132: 771-778.
12.- Savgaonkar MG, Chimmalgi M, Kulkarni, UK.
Anatomy of Inter-scalene Triangle and Its Role in Thoracic Outlet Compression Syndrome.
J Anat Soc India 2006; 55(2): 7-12.
13.- Poblete R.
Complicaciones neurovasculares del síndrome del opérculo torácico.
LXXIV Congreso Chileno e Internacional de Cirugía, Pucón, Chile, Noviembre 2002.
14.- Campbell PT, Simel DL.
Left arm pain isn´t always angina.
NC Med J 1988; 49: 564-567.
15.- Urschel HC, Razzuk MA, Hyland JW, Mantson JL, Solis RM, Wood R, et als.
Thoracic outlet syndrome masquerading as coronary artery disease (pseudoangina).
Ann Thorac Surg 1973; 16: 239-248.
16.- Selmonosky C.
Brachial entrapment neuropathies in the diagnosis of chest pains.
Presentation Annual Meeting Southern Medical Association 1999; Oct 14. Nashville, TN.
17.- Fernandez Noda EI, Rivera H, Perez J, Castillo J, Perez
M, Estrada C.
New concept regarding chest pain due to hypoxia of the internal mammary artery in more than 1600 operated patients with cerebral thoracic neurovascular syndrome (CTNVS).
Panminerva Med 2002; 44: 47-59.
18.- Selmonosky C, Byrd R, Blanc JS.
Useful triad for diagnosing the cause of chest pain.
Southern Med J 1981; 74: 947-949.
19.- Pistorius M, Planchon B.
Incidence of thoracic outlet syndrome on the epidemiology and clinical presentation of apparently primary Raynaud´s phenomenon.
Int Angiol 1995; 14(1): 60-64.
20.- Sell JJ, Rael JR, Orrison WW.
Rotational vertebrobasilar insufficiency as a component of thoracic
outlet syndrome resulting in transient blindness. Case report.
J Neurosurg 1994; 81: 617-619.
21.- Jung A, Kehr P.
Vertebral artery syndrome and scalenus syndrome.
J Chir (Paris) 1968; 95: 437-456.
22.- Kojima N, Tamaki N, Matsumoto S.
Vertebral artery occlusion at the narrowed "scalenovertebral angle": Mechanical vertebral occlusion in the distal first portion.
Neurosurg 1985; 16: 672-674.
23.- Bacquey F, Hamon M, Coskun O, Coffin O, Joidate A, Corthéoux P et als.
Rotational vertebro-basilar insufficience secondary to a fibrous bands of the longus colli muscle: Value of CT spiral angiography diagnosis.
J Radiol 2002; 83: 979-982.
24.- Kimura R.
Animal models of inner ear vascular disturbances.
Am J Otolaryngol 1986; 7: 130-189.
25.- Rob C, Standmen A.
Arterial occlusion complicating thoracic outlet compression syndrome.
Br Med J 1958; 2: 709-719.
26.- Desai Y, Robbs J.
Arterial complications of the thoracic outlet syndrome
Eur J Vasc Endovasc Surg 1995; 10: 362-365.
27.- Salo JA, Varstela E, Ketonen P.
Management of vascular complications in Thoracic Outlet Syndrome.
Acta Chir Scand 1988; 154: 349-352.
28.- Pedrini L, Pisano E, Sensi L, Isceri S.
Superior vena cava thrombosis secondary to thoracic outlet syndrome.
Int Angiol 2000; 19(4): 366-368.
29.- Selmonosky C, Poblete R.
Diagnóstico del síndrome del opérculo torácico. Tríada diagnóstica y el Signo de la mano blanca.
En: Poblete R. cirugiavascularactual.blogspot.com, Abril 18, 2010.
30.- Yannikas C, Walsh JC.
Somatosensory evoked response in the diagnosis of thoracic outlet syndrome.
J Neurol Neurosurg Psichiatry 1983; 46: 234-237.
31.- Wilbourn AJ, Porter JM.
Thoracic outlet syndromes.
Spine. State of the Art Rev 1988; Sept; 2(4): 597-626.
32.- Huang JH, Zager EL.
Thoracic outlet syndrome.
Neurosurg 2004; 55: 897-902, discussion: 902-903.
33.- Selminosky CA.
Thoracic Outlet Syndrome. The missing link in the diagnosis of non-coronary chest pain.
Italian J Cardiol 2008;Dec 9:(12) 217 S. Abstract
34.- Urschel HC, Kourtis H Jr.
Thoracic outlet syndrome: a 50 years experience at Baylor University.
Proc Bay Univ Med Ctr 2007 Apr 20; (2): 125-135.
35.- Brantigan CO, Ross DB.
Diagnosis thoracic outlet syndrome.
Hand Clin 2004 Feb; 20(1): 27-36.
36.- Glockner SM.
Shoulder pain. A diagnostic dilemma.
Am Fam Physician 1995; 51: 1677-1687 and 1690-1692.
37.- Eslick GD, Talley NJ.
Non-cardiac chest pain: Squeezing the life out of australian healthcare system.
Med J Aust 2000; 173: 233-234.
38.- Zamboni P, Galeotti R, Menegatti E, Malagoni, AM,
Gianesini S, Bartolomei I, et als.
A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency.
J Vasc Surg 2009; 50(6): 1348-1358.e3.
39.- Zamboni P, Galeotti R, Menegatti E, Malagoni AM et als.
Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis.
J Neurol Neurosurg Psychiatry 2009; 80: 392-399.
40.- Tobinick E, Vega CP.
The cerebrospinal venous system: anatomy, physiology, and clinical implications.
Med Gen Med 2006; Feb 22; 8(1): 53.
41.- Talbert D.
Raised venous pressure as a factor in multiple sclerosis.
Med Hypotheses 2008; 70: 1112-117
42.- Schelling, F.
Damage venous reflux into the skull or spine: relevance to multiple sclerosis.
Med Hypotheses 1986; Oct 21(2): 141-148.
43.- Menegatti E, Zamboni P.
Doppler haemodynamics of cerebral venous return.
Current Neurovascular Research 2008; 5: 260-265.
44.- Simka M, Kostecki J, Zaniewski M, Najewoski E, Hartel M.
Extracranial doppler sonographic criteria of chronic cerebrospinal venous insufficiency in the patients with multiple sclerosis.
Int Angiol 2010; 29(2): 109-114.
45.- Al-Omari MN, Roussan LA
Internal jugular vein morphology and hemodynamics in patients with multiple sclerosis.
Int Angiol 2010; 29(2): 115-120.
46.- Poblete R, Draper S, Suárez L.
Cirugía en el síndrome del opérculo torácico.
Rev Chil Cir 1987; 39 (4): 302-305.
47.- Poblete R.
Resultados de la cirugía del síndrome del opérculo torácico. Puntos críticos y reoperaciones.
Rev Chil Cir 2002; 54(6): 566-72.
48.- Meliere D, Becquemin J, Etienne G, et als.
Severe injuries resulting from operations for thoracic outlet syndrome: can they be avoided?
J Cardiovasc Surg 1991; 32: 599-603.
49.- Sanders R, Haugh C, Pearce W.
Recurrent thoracic outlet syndrome
J Vasc Surg 1990; 12: 390-400.
50.- Urschel H, Razzuk M, Albers JE, Wood RE, Paulson DL.
Reoperations for recurrent thoracic outlet syndrome.
Ann Thorac Surg 1976; 21: 19-25.
51.- Martinez N.
Posterior first rib resection for total thoracic outlet syndrome decompression
Cont Surg 1979; 15: 13-21.
52.- Roos D.
Transaxillary approach for first rib resection to relieve Thoracic Outlet Syndrome.
Ann Surg 1966; 163: 354-359.
53.- Selmonosky CA, Poblete R.
Requisites to be met before elective surgery for thoracic outlet syndrome (TOS),
TOS Syndrome surgery requirements (cited 2010 Aug 17) Available
www.tos-syndrome.com/old1/new_page_1.htm
54.- Redford JB.
Physical treatment.
In Pollak E. Thoracic Outlet Syndrome. Erich W Pollak; New York Ed Futura Publishing Co, New York, 1986: 151-168.